Article Text
Abstract
Preparticipation screening programmes for underlying cardiac pathologies are now commonplace for many international sporting organisations. However, providing medical clearance for an asymptomatic athlete without a family history of sudden cardiac death (SCD) is especially challenging when the athlete demonstrates particularly abnormal repolarisation patterns, highly suggestive of an inherited cardiomyopathy or channelopathy. Deep T-wave inversions of ≥2 contiguous anterior or lateral leads (but not aVR, and III) are of major concern for sports cardiologists who advise referring team physicians, as these ECG alterations are a recognised manifestation of hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC). Subsequently, inverted T-waves may represent the first and only sign of an inherited heart muscle disease, in the absence of any other features and before structural changes in the heart can be detected. However, to date, there remains little evidence that deep T-wave inversions are always pathognomonic of either a cardiomyopathy or an ion channel disorder in an asymptomatic athlete following long-term follow-up.
This paper aims to provide a systematic review of the prevalence of T-wave inversion in athletes and examine T-wave inversion and its relationship to structural heart disease, notably HCM and ARVC with a view to identify young athletes at risk of SCD during sport. Finally, the review proposes clinical management pathways (including genetic testing) for asymptomatic athletes demonstrating significant T-wave inversion with structurally normal hearts.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/3.0/ and http://creativecommons.org/licenses/by-nc/3.0/legalcode
Statistics from Altmetric.com
INTRODUCTION
Physiological remodelling of the athlete's heart has a significant impact on the resting 12-lead ECG. Sinus bradycardia, voltage criterion for left ventricular hypertrophy (LVH), first-degree atrioventricular (AV) block and incomplete right bundle branch block (RBBB) are common; other recognised electrical anomalies are relatively rare and raise the possibility of an underlying cardiac disorder. One of the most challenging conundrums for the sports cardiologist is the interpretation and management of profound repolarisation changes, notably deep T-wave inversions, which are also common manifestations of disorders implicated in exercise-related SCD among young athletes.
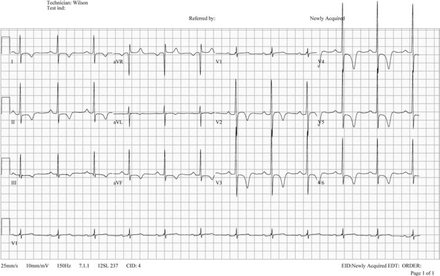
Recent case studies by Zeller et al1 and Wilson et al2 demonstrate the complex scenarios faced regularly by sports cardiologists in providing medical clearance for competitive athletes. Furthermore, for athletes displaying T-wave inversion despite strictly normal secondary investigations, the quantification of SCD risk in the absence of significant risk factors at the time of examination remains unknown. Guidelines from both the ACC 36th Bethesda Conference and European Society of Cardiology recommend that athletes with unequivocal or ‘probable’ cardiomyopathy abstain from competitive sport and vigorous training with the exception of low-intensity activities.3,4 However, in an asymptomatic athlete displaying T-wave inversion (figure 1) without a family history of SCD, who demonstrates normal further cardiac examinations, these guidelines differ in their management outcome for sporting clearance.
T-wave inversion in leads aVR, V1–V3 in a 12-year-old asymptomatic Middle-Eastern athlete without a family history of SCD (A), and T-wave inversion in leads aVR, V1–V4 in a 17-year-old asymptomatic mixed race (Middle-Eastern/Black African) athlete without a family history of SCD (B). Note; both athletes do not require further cardiological evaluation based on these ECG features.
This study aims to review the cardiology and sports medicine literature regarding the prevalence of T-wave inversion. For the purpose of this review, only T-wave inversions in ≥2 contiguous anterior, inferior or lateral leads (but not aVR, and III) were considered significant in athletes. An athlete is an individual of young and adult age, either amateur or professional, who is engaged in exercise training on a regular basis and who participates in official sports competition.4 Furthermore, it will examine T-wave inversion and its relationship to structural heart disease, notably hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) with a view to identify young athletes at risk of SCD during sport. Finally, based upon the group's combined experience, the review will propose clinical management pathways (including genetic testing) for asymptomatic athletes demonstrating marked T-wave inversion but with structurally normal hearts.
ECG MODIFICATIONS REFLECTING PATHOLOGY IN ATHLETES
Regular participation in physical training demands substantial and sustained increases in cardiac output and afterload. The physiological cardiac adaptation to chronic increases in preload and afterload on the heart results in a form of reversible cardiac remodelling including left (LV) and right ventricular (RV) hypertrophy, increases in cardiac chamber size and enhanced diastolic ventricular filling;5 so called ‘athletes heart’. Not only does the heart remodel structurally with prolonged intensive exercise, but athletes also demonstrate a spectrum of electrical alterations in the 12-lead ECG. These frequently include sinus bradycardia, first-degree AV block, early repolarisation and voltage criteria suggestive of LVH.6 Despite these observations, data from a number of international preparticipation screening programmes7–9 have identified a small but important number of athletes who demonstrate particularly abnormal ECG patterns, which are as yet unproven, but highly suggestive of an inherited cardiomyopathy.
PREVALENCE OF ECG REPOLARISATION MODIFICATIONS
Data from an unselected population of over 40 000 young (≤35 years) adult Italian caucasian athletes revealed that <5% exhibited T-wave inversion (excluding V1 and aVR).10 The prevalence and distribution of T-wave inversions are influenced by several demographic factors including age, sex and ethnicity. Specific electrocardiographic studies examining large numbers of highly trained athletes have demonstrated a T-wave inversion prevalence of 3% in caucasian adults11 and 4% in caucasian adolescent athletes.12 There are few reports on the effect of the athlete's sex on T-wave inversion. Studies by Bjørnstad et al13 found the prevalence of T-wave inversion in more than three contiguous leads was greater in athletes compared with sedentary controls of similar age but did not differ significantly between male and female athletes (0.8% vs 1.0%). Our personal experience of over 10 000 athletes (∼90% caucasian) aged between 14 and 35 years reveals a higher prevalence of T-wave inversion in males than females (4% vs 2%). Deep (>0.2 mV) T-wave inversions are rare in females and identified in less than 0.5% of all caucasian females. Indeed, we suggest that deep T-wave inversion in caucasian female athletes is extremely rare and should warrant investigation for an underlying cardiac disorder.
The ethnicity of an athlete also has a major effect on the prevalence of T-wave inversion. Athletes of African/Afro-Caribbean origin exhibit a significantly higher prevalence of T-wave inversion compared with caucasian's (23% vs 3%).14 Within the African/Afro-Caribbean group, the prevalence of T-wave inversion is considerably higher in males compared with females (23% vs 14%).15 Data relating to other ethnic groups are scarce; however, a recent study from the Middle-East in a largely male Arabic athletic population reveals a similar T-wave prevalence to caucasian athletes.16 Studies from our group examining T-wave inversion in adolescent and adult caucasian and black athletes have failed to identify any significant association between the presence of T-wave inversions and sporting disciplines. However, an inherent limitation of these studies is that they are confined to sporting disciplines with a high-participation rate between ethnicities. Therefore, endurance sports such as cycling, swimming and rowing, which have a low-participation rate among black athletes have not been examined in detail.
DISTRIBUTION OF T-WAVE INVERSION
Anterior T-wave inversion
Based on a study of 1700 highly trained athletes aged between 14 and 18 years, T-wave inversion in the anterior leads (V1–V3) are observed in 2.5% of male and female caucasian athletes aged <16 years and are considered to represent the juvenile ECG pattern (figure 2A) in the absence of symptoms, a previous history of an intracardiac shunt, a cardiac murmur consistent with an atrial septal defect or a family history of ARVC or premature SCD.11 T-wave inversion beyond lead V2 is exceptionally rare in caucasian athletes aged >16 years (0.1%) and are an indication for further investigation. In contrast, T-wave inversion in the anterior leads (V1–V4) is common in adult black athletes (figure 2B). A recent study examining 904 black athletes and 1819 white athletes aged 14–35 years participating in 22 different sporting disciplines revealed that T-wave inversions were present in up to 25% of athletes and half of these individuals exhibit deep (−0.2 mV) T-wave inversions.14 T-wave inversions in leads V1–V4 were usually asymmetric or biphasic and frequently proceeded by convex ST-segment elevation. In black athletes, T-wave inversion was only preceded by ST-segment elevation or isoelectric ST-segments but never ST-segment depression. Detailed evaluation of these athletes with echocardiography, exercise stress tests, cardiac MRI and 24 h Holter failed to demonstrate any of the broad phenotypic features of HCM or ARVC, and following an almost 7-year follow-up episode there were no adverse events in black athletes with T-wave inversions in leads V1–V4. The same study also revealed that black controls of similar age had a T-wave inversion prevalence of 10% mainly distributed in the anterior leads indicating the T-wave inversion in leads V1–V4 in black athletes may represent ethnic variation which is exaggerated by exercise. Support for this hypothesis was evidenced by the fact that detraining among several athletes resulted in resolution of T-wave inversions within a few weeks.
T-wave inversion in leads I, II, III, aVF, V2–V6 and ST-segment depression in leads II, aVF, V4–V6 in a 31-year-old asymptomatic professional football referee (Middle-Eastern) without a family history of SCD. Normal echocardiogram, late gadolinium-enhanced cardiac MR, cardiopulmonary exercise stress test, 24 h Holter and no T-wave inversion found in siblings.
Inferior and lateral T-wave inversion
T-wave inversion in the inferior and lateral leads is identified in 1.5–1.8% of adult and caucasian athletes. However, T-wave inversions in the lateral leads are confined to males and have been identified in only 0.3%.11,14,15 In contrast, T-wave inversions are identified in 10% of black athletes (6% inferior leads and 4% lateral leads) but as with caucasian athletes, T-wave inversion in the lateral leads is usually absent in black female athletes.
Although T-wave inversions in the lateral leads are identified in 4% of black athletes, we have observed two cases of aborted SCD in two young black football players with these repolarisation changes in the absence of a structural abnormality of the heart, indicating the presence of a subtle cardiomyopathy or an ion channel disorder. Comparison of the pattern of T-wave inversions in black athletes and black patients with HCM demonstrates that T-wave inversions in black HCM patients are usually confined to the lateral leads, being present in almost 80% of cases, whereas as T-wave inversion in leads V1–V4 is only observed in 3–4% of HCM patients compared with 12% of black athletes. More detailed genotype–phenotype studies are required in black athletes to elucidate the precise nature of marked repolarisation changes in the lateral leads.
EXAMPLES OF T-WAVE INVERSION IN ATHLETES WITHOUT PROVEN CARDIOMYOPATHY
Cardiac pathologies associated with T-wave inversion
HCM (figure 3) is characterised by the presence of increased ventricular wall thickness or mass in the absence of loading conditions (hypertension, valve disease, etc) sufficient to cause the observed abnormality.17 There are three important spectral ECG changes commonly observed within HCM; (1) the ECG is abnormal in approximately 90% of patients with HCM, (2) whereas voltage criteria for LVH are present in around 75% of patients with HCM, isolated Sokolow-Lyon voltage criterion for LVH commonly observed within athletes (without associated ST and T wave changes) occurs in only 2% of HCM patients, and (3) repolarisation changes consisting of ST segment shift and T-wave inversion are present in over 90% of cases. ARVC (figure 4) is a myocardial disease characterised by fibro-fatty replacement and ventricular arrhythmias.18 The fibro-fatty replacement interferes with electrical impulse conduction and is the key cause of T-wave inversion in the right precordial leads, ɛ waves, RBBB, late potentials and re-entrant ventricular arrhythmias.19 Brugada syndrome (figure 5) is characterised by a QRS complex resembling incomplete RBBB with J point elevation and ST-segment elevation in V1 through V3, often followed by a negative T-wave and a propensity for SCD typically occurring with mild activity or during sleep.20 It remains a diagnostic challenge to exclude Brugada syndrome in young athletes as features such as mild elevation of the J point associated with mild ST segment elevation in the anterior chest leads and mild T-wave inversion (<0.2 mV) in V1–2 and the inferior leads, together with incomplete RBBB are frequently observed.21
T-wave inversion in leads V1–V6 in a 38-year-old symptomatic (an episode of sustained monomorphic ventricular tachycardia, 250 bpm, with left bundle branch morphology) former soccer player with genotypically confirmed arrhythmogenic right ventricular cardiomyopathy and a family history of SCD (brother at 26 years of age).
T-wave inversion in leads II, III, aVF, V1–V6, ST segment depression in V4 and profound left ventricular hypertrophy voltage criteria in 27-year-old asymptomatic Middle-Eastern Futsal player with confirmed apical hypertrophic cardiomyopathy.
An ECG demonstrating typical type 1 Brugada syndrome.
Other causes of T-wave inversion
There are a number of alternative causes of T-wave inversion outside of HCM, ARVC and BrS that sports cardiologists should be aware of; (1) drug induced (ie, digoxin/cocaine/amphetamines), (2) acute pulmonary embolism, (3) myocardial ischaemia, (4) myopericarditis, (5) Takatsubo cardiomyopathy or Wellen's syndrome, (6) electrolyte disturbance/hypokalemia and (7) Long QT syndrome (particularly type 2).
Long-term outcome in athletes with T-wave inversions
Little long-term follow-up data exist for athletes demonstrating T-wave inversion. From a database of 12 550 athletes, Pelliccia et al9 reported on 81 athletes with diffusely distributed and deeply inverted T-waves who had no apparent cardiac disease and who had undergone serial clinical, ECG and echocardiographic studies for a mean (SD) of 9 ± 7 years (range 1–27). From the 81 athletes, 63 with an abnormal repolarisation pattern (78%) were still engaged in regular competition and training. During serial follow-up, ECG alterations remained essentially unchanged (or showed deepening of T-wave inversion) in 54 athletes (67%). In the remaining 27 athletes, ECG patterns either normalised completely (in 12) or became less abnormal (in 15). No changes in LV dimensions were observed in the 81 athletes during the follow-up period regardless of change in ECG patterns. A diagnosis of pathological cardiomyopathy was eventually made in 5 (6%) of the 81 athletes who had no previous evidence of cardiac disease (except deep T-wave inversion in the lateral leads), including two athletes who had undergone significant cardiac events (one death from clinically undetected ARVC and one aborted cardiac arrest).
While the long-term follow-up of Pelliccia et al9 was the first to suggest that these repolarisation abnormalities may represent the initial expression of underlying genetic cardiac disease, preceded by many years before other phenotypic features and adverse clinical outcomes, there are several limitations that should be addressed before drawing a firm conclusion. First, 75 athletes (94%) were not diagnosed with a cardiomyopathy or ion channelopathy after 9 years of follow-up. Second, clinical long-term assessment did not include any of the newer and more accurate techniques of tissue doppler imaging and strain echocardiography or late gadolinium enhanced cardiac magnetic resonance. Thus, the remaining population of 75 athletes with marked repolarisation abnormalities is simply a reflection of those individuals Pelliccia et al could not yet identify with an unexplained cardiac pathology rather than those genuinely excluded from having cardiac pathology and who will ultimately go on to phenotypically express a cardiomyopathy. While the mean number of follow-up years was 9, the range was exceptionally large (1–27 years). Finally, no mutational analysis for a cardiomyopathy or ion channelopathy was undertaken in any of the diagnosed and non-diagnosed athletes.
Papadakis et al14 examined 2723 athletes, of which 1243 athletes were followed for a duration of 69.7 ± 29.6 months, together with 52 black patients with confirmed HCM. During follow-up, three athletes were diagnosed with HCM. Athlete 1 (black football player) was diagnosed following an abnormal ECG showing deep T-wave inversions and ST-segment depression in the inferior and lateral leads in the context of asymmetric septal hypertrophy and a non-dilated LV cavity on echocardiography and cardiac MRI. Athlete 2 (black football player) exhibited T-wave inversions in the inferior and lateral leads with mild concentric LVH on echocardiography and cardiac MRI. These features were initially considered to represent ‘athlete's heart’ based on a peak-VO2 ≥120% of maximum predicted and the absence of the broad HCM phenotype. The athlete was retrospectively diagnosed with HCM after successful resuscitation from ventricular fibrillation arrest during a football match. Athlete 3 (white triathlete) also exhibited T-wave inversions in the inferior and lateral leads but had a structurally normal heart on echocardiography and cardiac MRI. The athlete demonstrated high peak-VO2 and normal Holter recording but was diagnosed with HCM following identification of the apical form of HCM in his mother and subsequent confirmation with gene testing which identified a myosin-binding protein C mutation in both individuals.
Papadakis et al14 supports observations from Pelliccia and colleagues'9 long-term investigation, whereby the majority of patients with HCM (77%) and all three athletes diagnosed with HCM during follow-up exhibited T-wave inversions in the lateral leads, indicating that such ECG repolarisation patterns should be viewed with caution in any athlete. The long-term follow-up of Pelliccia et al9 is the most significant contribution to the T-wave inversion debate. It eloquently demonstrates the necessity for long-term cardiac follow-up, particularly given improvements in diagnostic technology. Papadakis and colleagues'14 recent follow-up study demonstrates the need for a robust molecular genetics study investigating whether asymptomatic athletes with T-wave inversions but without a family history of SCD who demonstrate absolutely normal secondary investigations have a genetically confirmed cardiomyopathy and ion channelopathy (as in the case of the white triathlete).
Minimum cardiac examinations required in the presence of T-wave inversion
In order to plan optimal management and treatment strategies in athletes with a suspected cardiomyopathy or ion channelopathy, it is critical to establish the diagnosis and underlying aetiology. Subsequently, the minimal cardiac examinations required in the presence of T-wave inversion in asymptomatic athletes are thorough physical examination, personal symptom and family history questioning, resting 12-lead ECG, echocardiography (including tissue doppler imaging, strain and speckle tracking), late gadolinium-enhanced cardiac magnetic resonance (CMR), maximal cardiopulmonary exercise testing incorporating blood pressure response, 24 h Holter ECG (including one exercise training session), and where possible, 12-lead ECG and echocardiography of first-degree relatives (>10 years). It should also be re-affirmed that an athlete's cardiac status changes immediately upon the presentation of personal symptoms suggestive of cardiac disease, irrespective of previously normal cardiac investigations. Finally, it is briefly worth justifying the inclusion of CMR in the routine work up of these athletes rather than on a case by case basis. CMR provides a comprehensive assessment of both ischaemic and non-ischaemic cardiomyopathies providing detailed precise information on cardiac anatomy, function, tissue characterisation, epicardial and microvascular perfusion, valvular flows and coronary and peripheral angiography. Measurements of maximal wall thickness are highly accurate, as is the pattern definition of LV wall thickening (focal vs mild concentric) and unlike echocardiography, no geometrical assumptions need to be made about the ventricle.22,23 Indeed, in some regions of the LV chamber, the extent of hypertrophy can be underestimated by echocardiography compared with CMR;24–26 which from personal experience is not diagnostically helpful in excluding apical HCM in athletes with deep lateral T-wave inversions. Finally, LGE provides a sensitive tool for the detection of myocardial fibrosis, abnormalities not typically seen in physiological LVH, thus highlighting pathology.27–29
ROLE OF GENETIC TESTING FOR ATHLETES DISPLAYING T-WAVE INVERSION
When dealing with athletes with T-wave inversion, the clinician must address two important and distinct questions: (1) what is the potential underlying cardiac disease, and (2) what is the potential risk of SCD? As discussed, the earliest phase of a cardiac expression may be characterised by ECG abnormalities such as T-wave inversion, preceding the later development of other identifiable structural abnormalities.30 This early phase of the cardiac expression creates diagnostic challenges, while potentially having a significant risk of SCD.31 However, before genetic testing occurs and in order for the athlete to fully understand the ramifications of a positive or negative genetic result, our group suggests cardiologists should follow the 2010 ESC position statement on genetic counselling and testing in cardiomyopathies.32 In a patient with an overt cardiomyopathy, the yield of mutation identification is variable according to the disease: 50–70% in HCM and around 40% in ARVC.33,34 Failure to identify a recognised mutation does not exclude the diagnosis of a cardiomyopathy or ion channelopathy for three important reasons; (1) not all genetic regions are assessed, (2) current technology is not able to detect some forms of mutation (intronic cryptic splice sites, large genomic rearrangements, etc) and (3) a similar phenotype may possibly develop without a specific genetic constitution. In future, technological developments in mutation analysis will increase the number of genes that can be assessed: fast sequencing technology is entering the clinical arena allowing for rapid analysis of a wide range of candidate genes (via capture arrays), the full exome or even the whole genome. Nevertheless, the results will continue to pose interpretation challenges resulting from the lack of understanding about the pathogenicity of mutations (particularly single-nucleotide polymorphisms) and the clinical interaction of many genetic variations.
RISK STRATIFICATION AND ATHLETIC ELIGIBILITY IN ATHLETES WITH T-WAVE INVERSION
Guidelines from both the ACC 36th Bethesda Conference and ESC recommend that athletes with unequivocal or ‘probable’ HCM abstain from competitive sport and vigorous training with the exception of low-intensity activities.3,4,35,36 The definition of ‘probable HCM’ in an asymptomatic athlete whose only abnormality is T-wave inversion is worrying for many sports cardiologists; especially as sport disqualification is regarded by some cardiologists as the safest approach for these athletes until more data becomes available. Risk stratification for SCD in athletes suspected or diagnosed with a cardiomyopathy or ion channelopathy (ie, (1) asymptomatic athlete with ominous T-wave inversion; (2) asymptomatic genotype carrier; and (3) asymptomatic phenotypic expressions, like LVH, without genotype) remains challenging. This electrocardiographic suspicion is not helped by the fact that the ECG is abnormal but not diagnostic in almost all patients with HCM and 90% of patients with ARVC potentially result in a high-false positive rate. Accordingly, based upon the group's experience, this review will provide some management pathways for asymptomatic athletes presenting with T-wave inversion suggestive of a cardiomyopathy or ion channelopathy.
PROPOSED CLINICAL MANAGEMENT PATHWAY FOR ATHLETES WITH T-WAVE INVERSION
Asymptomatic athlete with T-wave inversion and a definitive diagnosis of cardiac disease after detailed cardiac evaluation: recommend cessation of competitive sports and offer genetic testing for potential family management.
Asymptomatic athlete with T-wave inversions but normal detailed cardiac evaluation and no family history of hereditary cardiac disease: recommend unrestricted participation to competitive sports but offer genetic testing. Inform and educate the athlete regarding the development of symptoms, and place under yearly cardiac evaluation. Furthermore, propose a systematic cardiac examination with 12-lead ECG and echocardiography of first degree-relatives (>10 years).
Asymptomatic athlete with T-wave inversion with mild cardiac abnormalities (inconclusive of a cardiomyopathy or ion channelopathy) but no family history of hereditary cardiac disease: recommend unrestricted participation to competitive sports but offer genetic testing. Inform and educate the athlete regarding the development of symptoms, and place under yearly cardiac evaluation. Furthermore, propose a systematic cardiac examination with 12-lead ECG and echocardiography of first degree-relatives (>10 years).
Asymptomatic athlete with T-wave inversion with mild cardiac abnormalities (inconclusive of a cardiomyopathy or ion channelopathy) and a family history of hereditary cardiac disease: recommend restricted participation to mild to moderate competitive sports but offer genetic testing. Inform and educate the athlete regarding the development of symptoms, and place under yearly cardiac evaluation. Furthermore, propose a systematic cardiac examination with 12-lead ECG and echocardiography of first degree relatives (>10 years). It is worth elaborating on example 4. Bayesian probability would suggest that if an athlete has ECG abnormalities and a parent with an autosomal dominant genetic disease, the probability that the athlete is genetically affected is increased. Most cardiologists would be highly suspicious of such athletes and would usually treat this athlete as if he/she were a known genetic carrier, regardless of whether the athlete refused genetic testing. Accordingly, our group cannot recommend unrestricted participation to competitive sports in athletes presenting such as example 4, as they carry an elevated, yet unquantifiable risk of SCD.
GENETIC TESTING OUTCOME CONSIDERATIONS
Question: what to do in an asymptomatic athlete with T-wave inversion with absolutely normal cardiac examination and no family history of SCD who refuses genetic testing?
Answer: allow full competitive activity but under yearly cardiac examination. Note: the athlete has the right to refuse genetic testing.
Question: what to do in an asymptomatic athlete with T-wave inversion with absolutely normal cardiac examination and no family history of SCD, who is gene positive for a cardiomyopathy or ion channelopathy?
Answer: this is a challenging situation for sports cardiologists, and is the most debated scenario among our group. Some in our group propose that if there are no personal symptoms or risk factors associated with sudden death, and no family history of premature sudden death, then mild to moderate sporting activity may be authorised but under regular (6 months) cardiac examination. However, if the athlete becomes symptomatic (ie, rhythm disturbance, syncope, etc), then full sporting disqualification must occur immediately. Others in our group are more conservative and propose full competitive disqualification immediately after the genetic result. Therein lies the problem, for which there is currently no uniform answer.3,4,35,36 In times of quandary, our group seeks a collaborative team approach (uneven number of at least three in case of vote), whereby other cardiologists with extensive experience in sports cardiology and inherited pathology are presented the case; making an independent decision before discussing the athlete as a group. This form of approach helps the overseeing cardiologist determine the final outcome, knowing that he/she has kept the athletes mental and physical well-being at the heart of the return-to-play or sporting disqualification verdict. Note however, that if the causal mutation was previously associated in other families with a worse prognosis, then full sporting disqualification may be discussed; cardiologists should seek guidance from specialist cardiac genetists in such cases.
Question: what to do in an asymptomatic athlete with T-wave inversion with absolutely normal cardiac examination and no family history of SCD, who is gene negative for a cardiomyopathy or ion channelopathy?
Answer: unfortunately, this result does not exclude the diagnosis of an inherited cardiac disease (as a mutation is identified in less than 100% of obvious patients with a cardiomyopathy). A management decision for competitive activity must be based without genetic data. Accordingly, allow full competitive activity, educate the athlete for personal symptoms and place under yearly cardiac examination.
CONCLUSION
It is well known that a minority of athletes will present with repolarisation abnormalities suggestive of an inherited cardiomyopathy or ion channelopathy that are recognised as a mechanism for SCD. The prevalence of T-wave inversion in this minority is also dependent on the athlete's age, sex and ethnicity and their interaction to prolonged and sustained physical exercise, with recent evidence suggesting that anterior T-wave inversion appears benign in adolescents and black athletes, while lateral T-wave inversion should always be viewed with a suspicion of disease in all populations. The differential diagnosis of ‘athletes heart’ versus ‘cardiac pathology’ is not helped by the fact that many athletes with T-wave inversion are asymptomatic, have no significant family history and demonstrate structurally normal hearts upon secondary evaluation. Furthermore, this conundrum is not improved when genetic testing returns ‘negative’ for a cardiomyopathy or ion channelopathy. It is possible that lateral T-wave inversions are benign,37 but the majority of evidence suggests that they may be part of a disease process and can be associated with normal cardiac structure and function that may later develop into pathology. Future research should utilise mutation analysis to examine the genetics of a large cohort of asymptomatic athletes without a family history of SCD who demonstrate absolutely normal secondary investigations in order to identify athletes at risk of developing a cardiomyopathy or ion channelopathy. In conclusion, T-wave inversions may be an important early harbinger of future disease and risk of SCD in a minority of athletes. It is essential that clinical follow-up of such athletes is undertaken by cardiologists with extensive experience in athlete's heart and inherited cardiac pathology, to ensure not only prompt diagnosis of underlying cardiac disease but also ensure a sensitive and balanced approach to minimise unnecessary investigations and anxiety which may have an effect on the athletes psychological well-being and performance.
Contributors MGW designed the review, MGW wrote the preliminary draft of the manuscript and all authors supplied comments and corrections, MGW and SS are the guarantors.
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.