Article Text
Abstract
Objective: In order to identify a reliable marker for the early detection of muscle injuries in sports, α-actin protein and other markers of muscle damage were studied in sera of uninjured sportspeople and those with skeletal muscle injury.
Methods: Blood samples were obtained from 20 sportspeople with skeletal muscle injury and 48 uninjured sportspeople. Immunoassays were performed to determine cardiac troponin I (TnI), troponin T, lactate dehydrogenase and myoglobin concentrations. Western blot and densitometry were used to measure α-actin concentrations. Skeletal muscle damage was diagnosed according to physical examination, MRI findings and the biochemical criterion of a creatine kinase value >500 IU/l (Rosalki method, Beckman Instruments SL, Fullerton, California, USA). Results were also compared with previously obtained data on injured and uninjured non-sportspeople.
Results: The mean serum concentration of α-actin was significantly higher in sportspeople with muscle damage (10.49 μg/ml) than in uninjured sportspeople (3.99 μg/ml). Sera from injured sportspeople showed higher levels of α-actin than of troponin or myoglobin. No significant difference in TnI levels was observed between the groups.
Conclusions: According to these results, α-actin is a new and reliable marker of skeletal muscle damage in sportspeople which can be used for the detection of muscle injury. Possible cross interference between skeletal and cardiac muscle damage can be discriminated by the combined use of α-actin and TnI. These data suggest that early measurement of α-actin in sportspeople with suspected muscle damage will allow them to receive earlier and more effective treatment and to return sooner to the practice of their sport.
- CK, creatine kinase
- LDH, lactate dehydrogenase
- MDA, malondialdehyde
- TnI, troponin I
- TnT, troponin T
Statistics from Altmetric.com
Muscle injuries are one of the most common traumas in sports1 and can be produced by intense or even moderate physical activity, especially eccentric exercise. It has been suggested that the immediate effects associated with muscle injury are mechanical, largely caused by the excessive tension to which the muscle sarcomeres are subjected.2,3 Intense physical exercise induces lesions in muscle fibre, whose severity depends on the duration and characteristics of the exercise, the training stage at which the sportsperson is found, and the presence of dietary or muscular (agonist/antagonist) imbalance.4 Although eccentric exercise has undoubted biomechanical and bioenergetic benefits, its intense or only occasional practice can produce structural and functional alterations of the muscles involved.5,6
Immunoassay is the most widely used approach in clinical biochemistry to identify and quantify molecules, and offers high sensitivity and specificity. Although some debate remains, the detection of proteins bound to intracellular structures (mitochondria, nucleus, etc), even in small amounts, is usually considered to indicate necrosis.7
The molecular diagnosis of muscle damage is largely based on measurement of the plasma activity of different sarcoplasmatic enzymes (creatine kinase (CK) and lactate dehydrogenase (LDH)). These enzymes are normally strictly intracellular, and their increased activity in plasma reflects their escape via membrane structures.8
Although the direct demonstration of muscle damage is histological, in practice the diagnosis is largely based on the measurement of plasma enzyme concentrations.8 Thus, the diagnosis can be supported by the combined measurement of biological and clinical parameters—for example, plasma LDH activity, myoglobin, malondialdehyde (MDA), number of leucocytes and changes in muscle parameters.9
The diagnosis of exercise-induced lesions in muscle by measurement of markers remains controversial. Thus, the presence of increased CK activity and MDA levels in plasma reflects only muscle overload and offers low specificity and sensitivity as a marker of muscle damage.10,11
Increasing attention has been paid to the clinical relevance of the detection of cellular proteins released after tissue injury, commonly referred to as biochemical markers, in plasma.12 The detection of actin13–15 and myosin molecules, closely related to muscle contraction, is of special interest and requires a reliable technique that offers high sensitivity and specificity. The aim of this study was to detect biochemical markers quickly and with high accuracy, especially when clinical and analytical findings fail to deliver an unequivocal diagnosis.
Our group detected previously, with a high sensitivity of 63.3–100%, the release of circulating α-actin in the serum of patients with known skeletal muscle damage, finding considerably lower levels of α-actin in uninjured individuals than in those with muscle damage.15 With this background, the present study was designed to test whether α-actin is a reliable, sensitive and specific marker of skeletal muscle damage in sportspeople in whom this type of lesion can be detected.
METHODS
Subjects
We studied 134 individuals with and without skeletal muscle damage, 68 sportspeople and 66 non-sportspeople. None of them had a history of cardiac problems. Professional sportspeople were recruited at the Andalusian Centre of Medicine and Sports and the non-sportspeople had been recruited at the Emergency Department of the Trauma and Rehabilitation Hospital, Granada, Spain, within 24 h of their trauma (road traffic accident). Skeletal muscle damage was defined by physical examination, MRI findings and a CK activity >500 IU/l (Rosalki method, Beckman Coulter, High Wycombe, UK).
After obtaining consent from participants, serum samples of approximately 5 ml were obtained at the above centres by nursing staff via venepuncture and collected in tubes with separator gel (Venoject II, Terumo Europe, Leuven, Belgium). The study was approved by the ethics committee of the centres. Four study groups were formed:
Non-sportspeople with skeletal muscle damage (group A): 33 patients with severe muscle traumas produced by contusions, accidents or falls, including 24 (72.7%) men with a mean age of 37.8 years and 9 (27.3%) women with a mean age of 39.2 years.
Sportspeople with skeletal muscle damage (group B): 20 patients, including 13 (65%) men with a mean age of 24.15 years and 7 (35%) women with a mean age of 23.4 years. The inclusion criterion was skeletal muscle lesion produced <2 days before its detection on MRI scan and by physical examination. Lesions involved the lumbar muscle, the most common localisation, femoral biceps, quadriceps, gluteus and ankle, among other sites. Their training load was 5 days/week. Of the patients, 10 were rowers and 10 were track athletes.
Sportspeople without skeletal muscle damage (group C): 48 individuals without known skeletal muscle damage or heart disease, including 42 (87.5%) men with a mean age of 28.6 years and 6 (12.5%) women with a mean age of 27.3 years. The training load was 5 days/week; 24 individuals of this sample played rugby and 24 played handball.
Non-sportspeople without skeletal muscle damage (group D): 33 individuals without known skeletal muscle damage or heart disease, including 24 (72.7%) men with a mean age of 54.5 years and 9 (27.3%) women with a mean age of 76.3 years.
Determination of total CK activity
The enzymatic kinetic method was used to determine total CK activity. In the reaction, CK catalyses the transfer of a phosphate group from creatine phosphate to adenosine diphosphate. Subsequent formation of adenosine triphosphate is measured by using two associated reactions, catalysed by hexokinase and glucose-6-phosphate dehydrogenase, which produce nicotinamide adenine dinucleotide. This CK assay contains the activator monothioglycerol.
Immunoassay determination of circulating troponin I, troponin T and myoglobin
Circulating cardiac troponin I (TnI), troponin T (TnT) and myoglobin levels were determined by chemoimmunofluorescence immunoassay. For TnI and myoglobin, an Access sandwich-type immunoenzymatic assay (Beckman Instruments) was used. A sample was added to a glass reaction vessel with alkaline phosphatase-conjugated anti-TnI and antimyoglobin monoclonal antibodies along with paramagnetic particles coated with anti-TnI and antimyoglobin monoclonal antibodies. Cardiac troponin and human myoglobin bind to the antibody in the solid phase, whereas the antibody–alkaline phosphatase conjugate reacts with different antigenic sites on cardiac troponin and myoglobin molecules. After incubation, the conjugate was separated in a magnetic field and washed to remove materials not bound to the solid phase. A chemiluminescent substrate, Lumi-Phos 530, was added to the reaction vessel and a luminometer was used to measure the light generated by the reaction. The production of photons is inversely proportional to the amount of enzymatic conjugate present at the end of the reaction and, consequently, to the concentration of cardiac TnI and myoglobin in the sample. The amount of analyte in the sample was determined by using a multipoint calibration curve. For TnT determination, the Elecsys 2010 TnT test (Roche Diagnostic, Indianapolis, Indiana, USA) was used, performed in 18 min at 37°C. In a first incubation step, a sandwich-type complex was formed by the sample, a specific biotinylated monoclonal antibody against TnT, and a ruthenium chelate-labelled (chelate tris(2,29-bipyridul)ruthenium(II), (Ru(bpy)2+3) specific monoclonal antibody against TnT.
Detection of α-actin by western blot
Sarcomeric α-actin was determined by western blot. A sample of serum (5 ml) from each study subject was dissolved in Laemmli’s sample buffer (62.76 mm Tris-HCl pH 6.8, 1% 2-mercaptoethanol, 1% sodium dodecyl sulphate, 10% glycerol and 0.01% bromophenol blue) at a 1:5 ratio, boiled for 5 min, microfuged for 1 min and analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in a Mini Protean II cell (Bio-Rad, Hercules, California, USA) at 60 mA for 1 h at room temperature. Gels with samples of serum were run in duplicate in all cases. Gels for immunoblot analyses were separated electrophoretically and transferred to a nitrocellulose membrane by applying a current of 20 V at room temperature for 30 min. Blots were treated with blocking solution (20 mM Tris, 0.9 NaCl, 10% non-fat milk) for 3 h at room temperature and then reacted with a 1:2000 dilution of anti-sarcomeric α-actin monoclonal antibody (Alpha-Sr-1 Clones, Dako, Glostrup, Denmark). Primary antibodies were incubated overnight at 4°C. Membranes were washed (15 min in 5% Tris-buffered saline with Tween) and incubated with horseradish peroxidase-conjugated goat anti-mouse IgM (whole molecule) conjugated with type VI horseradish peroxidase (1:1000, Santa Cruz Biotechnology, Heidelberg, Germany) for 1 h at room temperature, followed by additional washes (15 min in 5% Tris-buffered saline with Tween). Proteins were visualised by enhanced chemiluminescence (Bonus, Amersham, Little Chalfont, UK).
Densitometric analysis of α-actin
Densitometric analysis was performed by scanning radiographic images of membranes. Image resolution was 100 points per inch. Image treatment software (Adobe Photoshop 5.0, Adobe Systems) was used to treat images, which were stored in TIF format to allow them to be accessed by the software for quantification. This method yielded digital images of 10 wells: one for positive control (pure actin), another for molecular weight (Prestained SDS-PAGE Standard, Low Range, Bio-Rad Laboratories, Hercules, California, USA) and the remaining eight for the study samples. Quantity One 1-D Analysis Software for densitometric image analysis (Bio-Rad) was used to compare the different wells with the positive control. This method was used to calculate absolute α-actin values in all study samples.
Statistical analysis
In this quasi-experimental study (independent variable selected and not manipulated), a multiple analysis of variance (Mann–Whitney U test) or Kruskall–Wallis non-parametric test was used to compare marker levels among study groups. The statistical procedures were performed using the SPSS statistical package (V.11.5). Statistical significance was preset at the p<0.001 level.
RESULTS
Table 1 lists the means (SEM) of the study variables in each group. Results in non-sportspeople with (group A) and without (group D) skeletal muscle damage reported previously by our research group15 were compared with results in sportspeople with (group B) and without (group C) skeletal muscle damage recruited for the present study. Results of the earlier study are presented to offer a fuller view of how modifications in these parameters are produced.
Determination of levels of myoglobin, creatine kinase, lactate dehydrogenase, troponin T, troponin I and α-actin in serum
Serum myoglobin levels were significantly higher in injured sportspeople (group B) than in uninjured (group C) sportspeople. In contrast, CK activity was significantly higher in uninjured (group C) than in injured (group B) sportspeople and much higher in injured non-sportspeople (group A) than in either group of sportspeople. Significantly higher TnT levels were found in injured (groups B and C) than in non-injured (groups A and D) groups (table 2). No differences in LDH or TnT or I levels were found between the groups of sportspeople.
Results of the Mann–Whitney U test for the six markers in the paired group comparisons, indicating the Mann–Whitney U value and the p value
The Kruskal–Wallis test of comparison between pairs of groups was significant for the six muscle markers (χ23(myoglobin) = 80.861, p = 0.001; χ23(CK) = 93.951, p = 0.001; χ23(LDH) = 97.835, p = 0.001; χ23(TnI) = 12.172, p = 0.007; χ23(TnT) = 50.268, p = 0.001; χ23(ACT) = 102.979, p = 0.001). Analysis of differences between each pair of groups showed that only CK and α-actin discriminated among all study groups (table 2). Figure 1 shows the descriptive analysis of the data, which shows the mean for each marker in each group. All markers were scaled from 0 to 100 to enable visual comparisons.
Means of variables across the four study groups. CK, creatine kinase; LDH, lactate dehydrogenase; MYO, myoglobin; Tn, troponin.
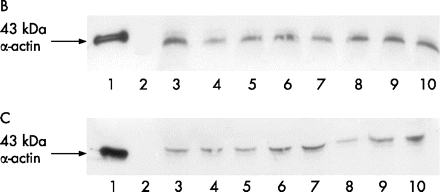
Western blot study of α-actin in sera of the sportspeople showed significant (p<0.001) differences between the uninjured and injured groups (fig 2). Densitometric analysis showed significant differences (p<0.001) in mean α-actin levels between the non-sportspeople with muscle damage and the sportspeople, either injured (10.49 μg/ml) or uninjured (3.99 μg/ml; table 1).
(A) Immunoblotting determination of α-actin levels in sera of sportspeople with a band at 43 kDa. (B) Number 1 corresponds to positive control (80 ng/ml of pure α-actin), number 2 corresponds to molecular weight and numbers 3–10 to α-actin levels in sera of sportspeople with skeletal muscle damage. (C) Number 1 corresponds to positive control (80 ng/ml of pure α-actin), number 2 corresponds to molecular weight and numbers 3–10 to α-actin levels in sera of uninjured sportspeople.
DISCUSSION
The clinical expression of exercise-induced muscle lesions is non-specific, considerably hampering assessment of their impact on the sports field.11 Moreover, the most widely used biochemical markers of muscle damage—that is, the plasma activity of muscle-specific enzymes such as CK and LDH11,16—have shown inadequate sensitivity, reproducibility and specificity. In this study, different indicators of muscle damage were examined to identify a reliable and specific marker for early detection of this type of lesion.
Increased myoglobin levels were found in non-sportspeople with skeletal muscle damage. Several authors have described this protein as a non-specific marker since it is found at both skeletal and cardiac levels and is released early into the blood.17 The more rapid appearance of myoglobin in blood may be a consequence of its lower molecular weight compared with the other proteins (17 kDa vs 80 kDa for CK) and its more direct route in the microvascular endothelium. Recent studies indicated that a combination of myoglobin with CK-MB and the troponins may represent a good marker of heart damage but not of skeletal muscle damage.18 Myoglobin levels within the normal range19 were found in the present study, with the exception of the non-sportspeople with skeletal muscle injury. Although higher myoglobin levels were shown in injured than in non-injured sportspeople, they were both considerably lower than those observed in injured non-sportspeople. The lowest myoglobin levels were found in the uninjured non-sportspeople. As pointed out by Clarkson and Hubal19 and others, muscle proteins such as myoglobin are released and appear in the blood of individuals involved in continual exercise, who show higher levels, regardless of the intensity of the exercise, than individuals who are not involved in continuous exercise.20
CK levels in the uninjured sportspeople (group C) were higher than in the uninjured non-sportspeople (group D) and even higher than in the sportspeople with skeletal muscle injury (group B). These findings may be explained by the influence of different variables on CK activity, such as oestradiol levels,21,22 daily training,23 sex24 or type of sports discipline.25,26 The fact that total CK levels were higher in uninjured than in injured sportspeople indicates that this parameter is not a good marker of skeletal muscle damage. Since samples were extracted at rest, the high CK activity in the uninjured sportspeople (group C) may be because of their previous training or the stage of their season, as proposed by Balnave and Thompson.23 High CK levels cannot specifically indicate skeletal muscle damage because of the presence of CK isoforms at skeletal, cardiac and brain levels (CK-MM, CK-MB and CK-BB) that are included in total CK activity.
Significant differences in LDH levels were observed between the groups with skeletal muscle damage and the uninjured non-sportspeople and between the latter group and the two groups of sportspeople (injured and uninjured). The much higher LDH levels shown by the sportspeople in samples taken at rest are in agreement with reports by Armstrong,27 who observed 100% higher LDH levels in sportspeople (260 IU/l) than in non-sportspeople (130 IU/l). Nevertheless, LDH is a non-specific marker of skeletal muscle damage because of its wide tissue distribution.11,16 Moreover, haemolysis during extraction or handling of the blood can produce a high LDH result.28 Some authors demonstrated that high LDH levels in trained sportspeople may possibly indicate chronic liver damage, since this organ works at a high rhythm during sports practice.29
Significant differences in TnT levels were found between injured and uninjured non-sportspeople and between injured and uninjured sportspeople. However, the levels of this protein were very low, thus the level detected in injured sportspeople (0.047 (0.017) ng/ml) cannot be considered to indicate muscle damage. In fact, higher TnT levels (0.06 ng/ml) were reported in healthy blood donors.30 In two recent studies, the mean plasma TnT concentration in healthy individuals was 0.001 ng/ml (range 0.00–0.02 ng/ml)31 and 0.0002 (0.001) ng/ml.32 Accordingly, if TnT levels were taken as the criterion, none of the present study groups would show muscle damage. Finally, TnT is a non-specific marker of muscle damage because both cardiac and skeletal isoforms are detected.
Minimal differences in TnI were observed among study groups, and no significant differences were found between injured and uninjured groups. According to Mair et al,33 this would be because of the cardiospecificity of TnI. In fact, this marker can be used to rule out possible cardiac muscle damage that might interfere with results, enabling differentiation between cardiac and skeletal lesions. Similar data have been published by various researchers, confirming the cardiospecificity of TnI and its lack of utility as a marker of skeletal muscle.34 The low level of TnI in the sera of all of our participants is an important finding, since it rules out any possible interference by heart damage in the detection of skeletal muscle damage in our series. Although significant differences were found between the injured non-sportspeople and uninjured sportspeople, they are of little relevance because the mean levels were very low (0.03 and 0.017 ng/ml). The reference values for the diagnosis of heart damage are 3.1–19 μg/l, with a sensitivity of 90–100% and a specificity of 93–100%.35 TnI values >40 ng/ml are associated with motility disorders of the ventricular wall and the appearance of Q wave on the ECG, and values >60 ng/ml are strongly associated with cardiac ischaemic events and arrhythmias.35
Serum α-actin levels were much higher in the uninjured sportspeople than in the uninjured non-sportspeople. This finding contrasts with the report by Féasson et al36 that exercise does not induce variations in the level of this protein. Our results suggest that the presence of circulating α-actin in the bloodstream may reflect cell lesions in patients with skeletal muscle damage.15 Significant differences were also found between the uninjured and the injured sportspeople. Much larger amounts of α-actin were detected in the sera in comparison with the other proteins. The mean level in individuals with muscle damage was 37.48 μg/ml, considerably higher than levels of the other markers. This finding indicates the specificity of α-actin to detect lesions in muscle fibres.
What is already known on this topic
-
The diagnosis of exercise-induced muscle lesions by measurement of markers remains controversial.
-
Thus, the presence of increased creatine kinase activity and malondialdehyde levels in plasma reflects only muscle overload and offers low specificity and sensitivity as a marker of muscle damage.
-
The detection of biochemical markers quickly and with high accuracy has great importance, especially when clinical and analytical findings fail to deliver an unequivocal diagnosis.
-
Our group previously detected, with a high sensitivity of 63.3–100%, the release of circulating α-actin in the serum of patients with known skeletal muscle damage, finding considerably lower levels of α-actin in uninjured individuals than in those with muscle damage.
What this study adds
The results presented in this paper indicate that α-actin is significantly increased in the serum of sportspeople with skeletal muscle damage in lumbar muscle, femoral biceps, quadriceps, gluteus and ankle, showing higher levels than other routinely used biochemical parameters.
Since no specific antibody is available to detect only the skeletal muscle isoform, Tn I had to be used to rule out possible heart damage. This lack of a specific antibody is largely due to the 98.9% homology between the protein sequences of the two α-actins, which only differ in two amino acids. As a result, although antibodies against sarcomeric α-actins can discriminate smooth muscle, they cannot distinguish between cardiac and skeletal muscle.
These results indicate that α-actin is significantly increased in the serum of sportspeople with skeletal muscle damage (in the lumbar muscle, femoral biceps, quadriceps, gluteus and ankle), showing higher levels than other routinely used biochemical parameters. However, this study was based on a small sample and further research is required to confirm these novel findings. Western blot is a highly sensitive and specific method for detecting α-actin in the serum. The combined use of α-actins and TnI allows possible cross interference between skeletal and cardiac muscle damage to be discriminated. Therefore, α-actin seems to be a new and reliable marker of skeletal muscle damage in sportspeople, which can be used for the early detection of muscle lesions to establish a more rapid and effective treatment.
Acknowledgments
This study was supported by the Andalusian Sports Medicine Center (CAMD) through project number 2003/319563.
REFERENCES
Footnotes
-
Published Online First 22 February 2007
-
Competing interests: None declared.